How Oil-Soluble Cleaners Can Redefine Lubricant Degradation Management

The mention of lubricant varnish in an industrial setting frequently evokes concerns regarding unscheduled equipment shutdowns, reliability challenges, and consequent extended downtime. However, a facet that often remains overlooked is the concept of ‘solubility.’
This underpins an understanding of how degradation products interact with lubricating oils. The idea of solubility, although seemingly esoteric, is central to devising robust strategies for lubricant degradation monitoring and management, thereby preemptively addressing varnish-related challenges and promoting operational excellence.
Understanding Solubility
Solubility is a fundamental chemical property referring to the ability of a substance (solute) to dissolve in a solvent to form a homogeneous solution at a particular temperature and pressure. Various factors, including the nature of the solute and solvent, temperature, pressure, and the presence of other substances, govern the extent of solubility.
Understanding solubility is crucial in industrial lubrication, especially regarding the behavior of oil degradation products within lubricating oils. As oils age, they undergo oxidative degradation and thermal breakdown, generating various degradation products like acids, aldehydes, ketones, and varnishes. The solubility of these degradation products in the lubricating oil will impact the fluid’s performance. Therefore, a thorough understanding of solubility principles and their application can significantly contribute to effective lubricant degradation monitoring and management in industrial settings.
Is It Soluble or Insoluble?
Oil degradation products are often considered soluble or insoluble. However, solubility can only be defined when associated with a certain temperature. Labeling degradation products as either soluble or insoluble can be an oversimplification, creating confusion. To clear up some of the misnomers about solubility, here are a few things to keep in mind:
- Varnish is insoluble while a machine is operational. If it were soluble, it would go back into solution. This doesn’t mean that varnish can never be dissolved. For example, if you increase the in-service oil’s temperature or its solubility using a solubility enhancer, varnish may become soluble and be dissolved into the oil.
- Oil degradation products are soluble when you obtain an oil sample. Oil degradation products can come out of solution once the sample is stored at room temperature. The exception is colloidal suspensions of degradation products, such as the carbonaceous soot particles produced in a diesel engine or a microdieseling process. Colloidal suspension refers to the dispersion of fine particles within the lubricant, where the particles are dispersed evenly but not dissolved. In this state, the particles remain suspended throughout the lubricant due to their small size (typically between 1 and 1000 nanometers) and interaction with the surrounding fluid.
- The MPC test (ASTM D 7843) measures soluble degradation products. That is, the degradation products are soluble while in the operating fluid. During the MPC test, the sample is heated to 65 °C for 24 hours to “reset” the sample. This dissolves any degradation products that may have settled out during shipping and handling. Then, the sample is stored at room temperature for 72 hours to provide a standardized time for the soluble degradation products to come out of solution. The exception is when colloidal suspensions are present from microdieseling events, which can be isolated with the 0.45mm membrane. (In this case, the color on the patch is black rather than the brown hues associated with oxidized degradation products.)
- There is no such thing as varnish in your oil. Varnish is defined as oil-derived deposits. If it is dissolved back into the oil, it is no longer varnished, as it has transitioned back to oil degradation products.
The Aniline Point
Group II basestocks are said to have less solvency than Group I basestocks. The test most often used to measure this is Aniline Point (ASTM D611), which gauges the solvency properties of lubricating oils. It refers to the minimum temperature at which a specific volume of aniline (a polar aromatic amine) will completely dissolve in a given volume of the lubricating oil to form a clear, homogenous solution under specified test conditions. A lower Aniline Point suggests that the lubricating oil has a higher aromatic content and, hence, a higher solvency capacity. Conversely, a higher Aniline Point denotes lower aromatic content and solvency.
Here are some average aniline points for various base stocks:
Naphthenic 58 °C
Group I 100 °C
Group II 116 °C
Group III 125 °C
Group IV 127 °C
Alkylated Naphthalene 42 °C
Polyalkylated Glycol -20 °C
However, understanding the solubility of oil degradation products is more involved than simply understanding the oil’s ability to dissolve a polar constituent. It depends upon the chemistry of the degradation products and the characteristics of the oil. Polarity is just one of the parameters. A more complete understanding of the solubility of oil degradation products can be determined by studying the Hanson Solubility Parameters.
What are Hansen Solubility Parameters?
Hansen Solubility Parameters (HSP) provide:
- A numerical representation of the molecular interactions in solutions.
- Enabling a more robust understanding of solubility.
- Miscibility.
- Dispersion phenomena.
They are three values derived from a particular material’s dispersion forces, polar forces, and hydrogen bonding interactions. The three parameters are often represented as a three-dimensional coordinate (D, P, H), each depicting different forces as outlined below:
- Dispersion Forces (dd):
This parameter represents the interactions due to van der Waals forces among molecules. It quantifies the non-polar interactions in a substance, often related to the London dispersion forces resulting from instantaneous dipole moments within molecules.
- Polar Forces (dp):
This parameter accounts for the dipolar interactions within and among molecules. It quantifies the polar interactions in a substance that arise due to permanent and induced dipoles.
- Hydrogen Bonding Forces (dh):
This parameter represents the potential for hydrogen bonding within a material. It quantifies the interactions arising from hydrogen bonding, a specific dipole-dipole interaction between hydrogen atoms and electronegative atoms like oxygen or nitrogen.
One can predict whether the solute would dissolve in the solvent by analyzing the Hansen Solubility Parameters of a solute and a solvent. Materials with similar HSP values are likely to be miscible or soluble with one another. This set of parameters can be applied to predict when oil degradation products may no longer be dissolved in oils, or when varnish can be redissolved back into the oil.
Moreover, HSP values can be graphically represented in a spherical three-dimensional Hansen Space to visualize the solubility and miscibility relationships among various materials. This is a powerful tool to develop solubility enhancers to manage oil degradation products.
If the chemistry of the degradation products, as defined by D, P, and H, creates an area inside that defined by the lubricant, the degradation products will dissolve. If it is outside the area, it will not dissolve.
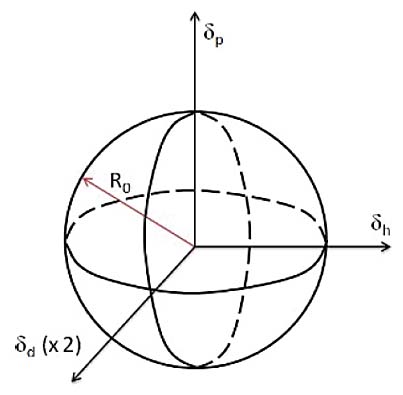
Figure 1: Hansen sphere made up of the three axes which can be used to understand the solubility of oil degradation products
Oil degradation products are soluble. This means that they can easily transition in and out of solution based on the equilibrium between the solute (varnish) and the solvent (oil) as seen in the varnish lifecycle in Figure 2.
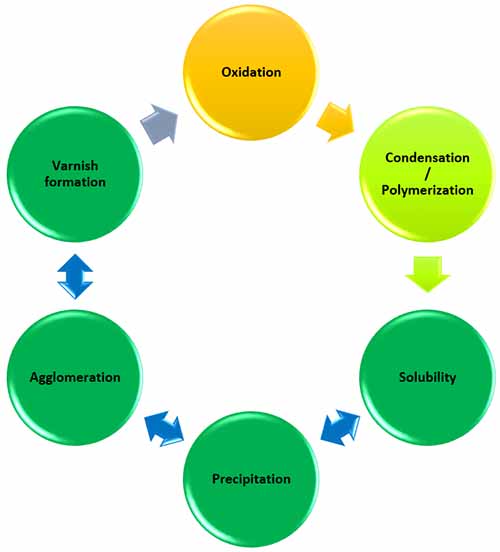
Figure 2: The Varnish Lifecycle showing the typical stages of oxidation to varnish formation.
The solubility of oil degradation by-products is significantly influenced by temperature. Analogous to the enhanced solubility of sugar in hot water as opposed to cold water, a rise in temperature increases the solubility of varnish in lubricating oil.
Technically, this is due to the elevated kinetic energy of molecules at higher temperatures, which facilitates the overcoming of intermolecular forces that otherwise impede solubility, thereby enabling the dissolution of more varnish.
Conversely, as the temperature diminishes, the kinetic energy of the molecules decreases, lessening the oil’s capacity to maintain the varnish in a dissolved state. This thermodynamically driven reduction in solubility during cooling promotes the precipitation of varnish from the solution, leading to its deposition on the internal surfaces of the equipment.
This phenomenon underscores the crucial role of temperature management in mitigating varnish-related complications in lubrication systems.
The Sandpaper Effect
Varnish can be complex structures of oxidation products and insoluble contaminants, such as wear metals and dirt. (In the strictest sense, even wear metals and dirt can dissolve in the lubricant under the right conditions, like water dissolving rocks as a river flows over them.)
Dr. A. Sasaki described this phenomenon as the Sandpaper Effect. The sticky nature of the oxidatively-derived varnish may allow suspended particles to stick to it, becoming trapped in the varnish. This type of varnish layer forms asperities on the surface.
Thus, when other surfaces encounter this type of varnish, they are susceptible to wear. This leads to the sandpaper effect, which ultimately damages the internals of the equipment, especially close tolerance components such as valves.
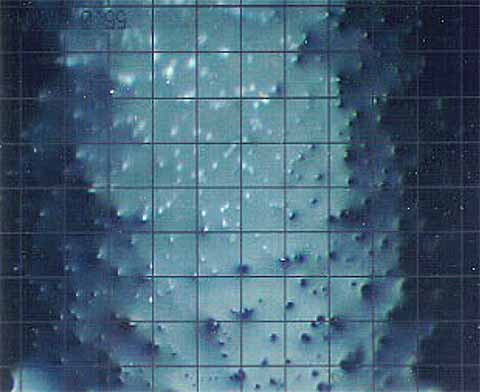
Figure 3: Microscopic image of a varnished “sandpaper” surface comprising soluble and insoluble deposits (Source A. Sasaki).
What Is the Role of Solubility Enhancers?
Solubility Enhancers can be a powerful technology to manage oil degradation products. They may be used to prevent oil degradation products from coming out of solution to form deposits or to dissolve varnish and keep it back in solution.
For a Solubility Enhancer to be deemed an effective solution, it should meet the following conditions:
- Miscible in lubricant – this will allow it to be added to an in-service lubricant during machine operation without specialized blending equipment.
- Wide range of compatibility – this extends to both in-service oils and system materials such as seals and paints
- No impact on an in-service oil’s characteristics – it should not interfere with the oil’s ability to interact with contaminants such as air and water. This can severely impact the oil’s air release, foam, and demulsibility characteristics.
- No impact on surface-active additive ingredients – this could potentially interfere with an oil’s corrosion inhibitors, antiwear, extreme pressure, or friction modifier additive systems.
- Engineered to solubilize oil degradation products effectively – this can give them the ability to hold a large amount of degradation products without the concern of these precipitating out under oxidative stress.
- Ability to work effectively without a varnish mitigation technology installed. This enhancer should ideally stand on its own.
- Ashless technology should be formulated without additive metals to ensure that it doesn’t contribute to varnish formation even under the most extreme oxidative conditions and without antioxidants.
Ideally, a solubility enhancer should add value to a system by removing varnish or keeping oil degradation products in solution without compromising the integrity of the lubricant or system.
Solvancer® – the Solubility Enhancer of the Future
Solvancer (a patent-pending technology) was developed using a blend of specialized synthetic API Group V chemistries. This technology has outstanding solubility characteristics and is compatible with many base oils and fully formulated lubricants.
It doesn’t impact system materials such as seals, filters, or paint and has excellent oxidation stability and long-term deposit control characteristics. Solvancer does not cause any adverse impact on fluid properties, nor does it use surface-active chemistry.
Solvancer was used in these two applications below, and the MPC results are definitive.
For this first example, 5% of Solvancer was added to a PAO Compressor oil, and the MPC value was lowered from 48 (patch on the left) to 6 (patch on the right).

Figure 4: MPC of a PAO compressor oil lowered from 48 to 6 with the use of Solvancer®
In this second example, Solvancer was added to a Group II-based Air compressor lubricant, and the MPC value was lowered from a rating of 67 to 11.

Figure 5: MPC of a Group II Air compressor changes from 67 to 11 with the addition of Solvancer®.
Not All Oil Soluble Cleaners are Created Equally.
There are now multiple oil-soluble cleaners available in the market. These chemistries may effectively dissolve varnish immediately before an outage to prevent system decontamination or oil flushing between oil exchanges. However, some chemistries may have unintended consequences if left in the system for too long.
To estimate the long-term performance of different solubility enhancers, they were mixed with new oil and subjected to the oxidative stresses in the Turbine Oil Performance Prediction (TOPP) test. This test procedure stresses the oil according to ASTM D7873 for 12 weeks.
Although many physical and chemical tests are done throughout this experiment, simply looking at the patch and condition of the glassware after the experiment tells a powerful story. The comparison of DECON versus two other commercially available solubility enhancers can be seen below in Figure 6.
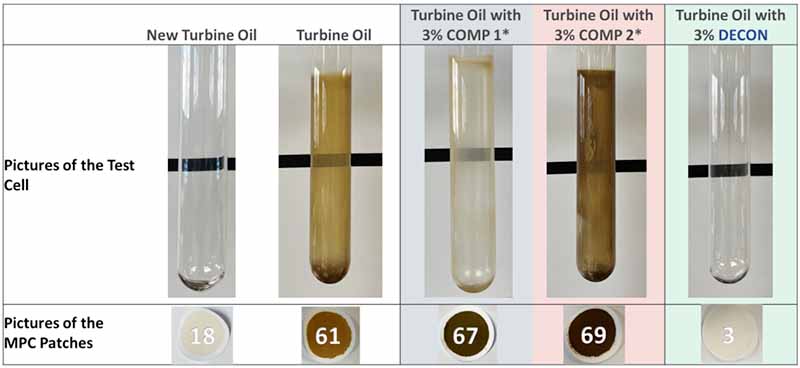
Figure 6: Benchmark comparison of two commercially available oil-soluble cleaners and DECON after 12 weeks in the TOPP experiment. This can be compared to new oil without the addition of solubility enhancers
In this case, a new, untreated turbine oil sample produced heavy glassware deposits and an MPC of 61 after the test. The same turbine oil was treated with 3% of COMP I, COMP 2, and DECON. The DECON-treated fluid maintained deposit-free, pristine glassware and a low MPC value, as seen above.
It should be noted that COMP 2 produced even more deposits than the untreated sample. Performance tests such as this must be carried out before using oil-soluble cleaners beyond a short period, immediately before an outage.
Available Technologies
Solvancer was formulated by utilizing Hansen’s Solubility Parameters to ensure the technology is optimized for long- and short-term deposit control. The technology allows deposits to remain in solution, even when the oil cools. It is incorporated into the following Fluitec products to provide deposit control:
Ideally, one must first understand their solubility when determining methods for managing oil degradation products. As outlined in this article, the answer does not lie in one simple test but rather in further exploration of the actual characteristics of the varnish and the system in which it is being produced. Solubility enhancers can be optimized for deposit control where they do not compromise the other characteristics of the oil.
