Why is LSV Used for RULER Analysis?
LSV represents Linear Sweep Voltammetry and is the method used in the popular RULER® (Remaining Useful Life Evaluation Routine) test. This voltammetric analysis is an electro-analytical method used to determine the amount of antioxidants in a lubricant. The results are based on current voltage and time relationships at the 3-electrode sensing system. This electrode system consists of a small, easily polarized microelectrode and a larger non-polarizable reference electrode.
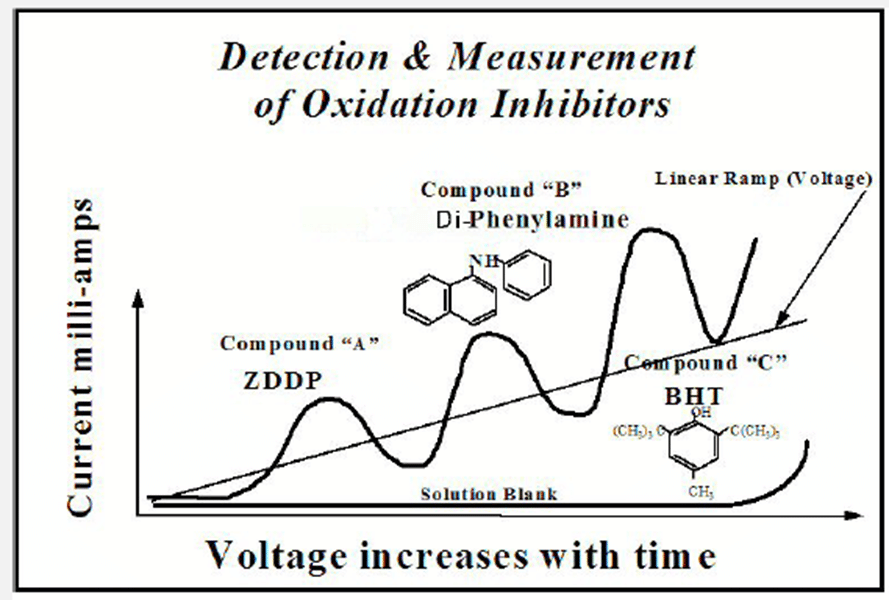
When voltammetric analysis is performed, the potential across the electrodes varies linearly with electrical sweep time. The resulting current is recorded as a function of the electrode potential. As the applied voltage increases to the cell, the dissolved antioxidants oxidize electrochemically. Therefore, they lose an electron based on their oxidation potential – resulting in an oxidation reaction which can be used to predict the remaining useful life of the lubricant.
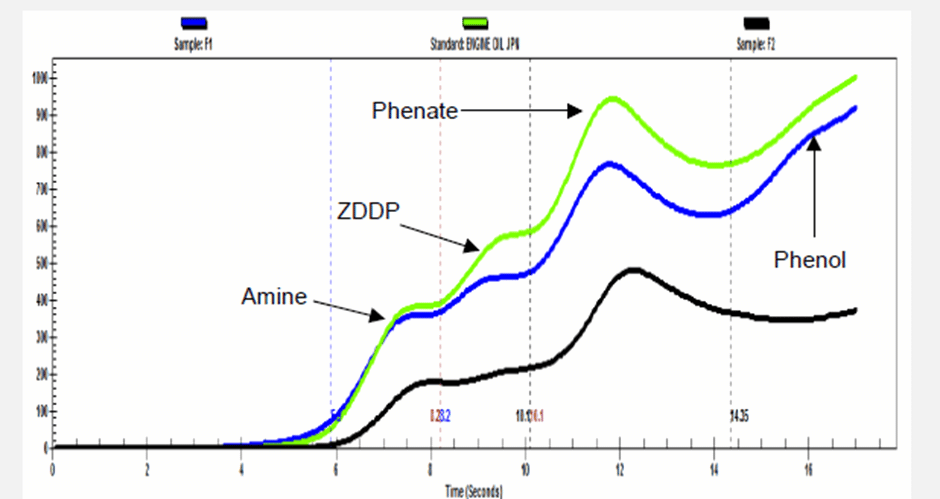
ASTM test methods D6810, D6971 and D7520 all describe the voltammetric method which is performed with the commercially available voltammograph instrument from Fluitec called the RULER®. As shown in the graph below, various antioxidants appear at different times.
Determining the correct solution
When preparing the used oil sample for the RULER test, it is important to choose the correct electrolyte solution. Electrolytes act as the carriers for the electric current to the different types of antioxidants. This allows them to be effectively oxidized at the carbon surface. As such, they can be easily detected by the voltammetric analyser. Fluitec has 4 solutions which are differentiated by their colours and applications.
Essentially, there are three types of electrolytes; neutral, basic and acidic.
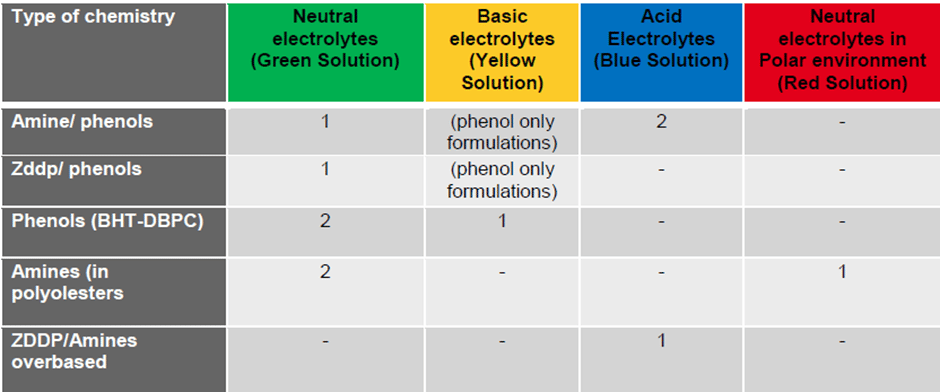
- Neutral electrolytes (Green or Red solutions) are strong electrolytes for amines, ZDDP and phenols detection.
- Basic electrolytes (Yellow solution) is really applied for the detection of phenolic antioxidants by deprotonating the hydroxyl group first which causes the response to move the oxidation potential. This process changes the position viewing location of the amine and phenol antioxidants with respect to the neutral electrolyte. The ZDDP oxidation potential is moved out of the viewing range with this electrolyte.
- Acidic electrolytes (Blue solution) is applied specifically for overbased formulations such as engine oils containing phenates and salicylates. In this case the overbasing is reacted to leave the original phenol to be analyzed. This electrolyte mixture can be used to detect ZDDP, phenols and amines.
The Table below summarizes a selection of available formulation chemistries and their selection of the electrolytic testing solutions. In this table, ‘1’ denotes that this is the recommended test solution and ‘2’ suggests that this test solution can provide some supporting information.
Detecting other electrochemically active compounds
This voltammetric technique is not only used for amines and phenols. It can also detect other electrochemically active compounds such as degradation products in mineral oils or phosphate esters, polymers, salicylates, phenates or even carbamates.
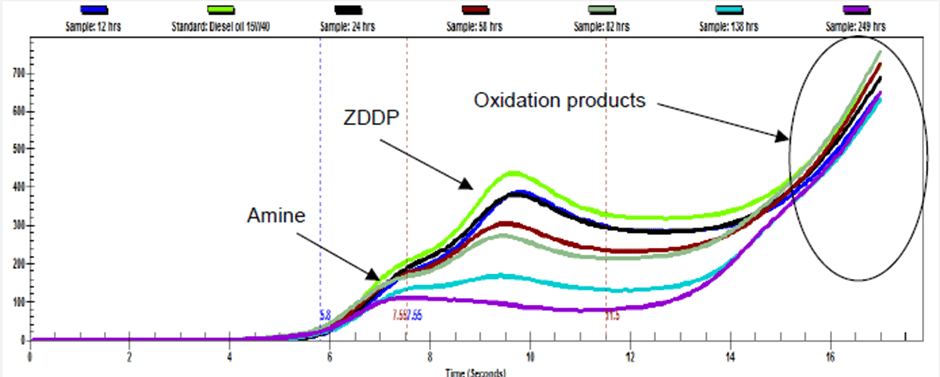
When mineral oils degrade, oxidation products can be produced. Typically, these are seen as the voltammetric tail in the RULER graph as shown below.
On the other hand, in phosphate esters, the ingress of water and increases in temperature promote hydrolysis which causes these fluids to produce di-aryl acid phosphate (also called mono acid phosphate). As the acid concentration increases this acid can be further hydrolysed to aryl di-acid phosphate (also known as dihydrogen mono aryl phosphate). Even further hydrolysis can produce phosphoric acid.
Each acid produces a corresponding alkyl-phenol. The RULER test can determine the quantity present of these alkyl-phenols. These negatively affect the air release, resistivity and will transition into an insoluble state eventually. Hence monitoring of these phenol degradation products can help determine the condition of the lubricant.
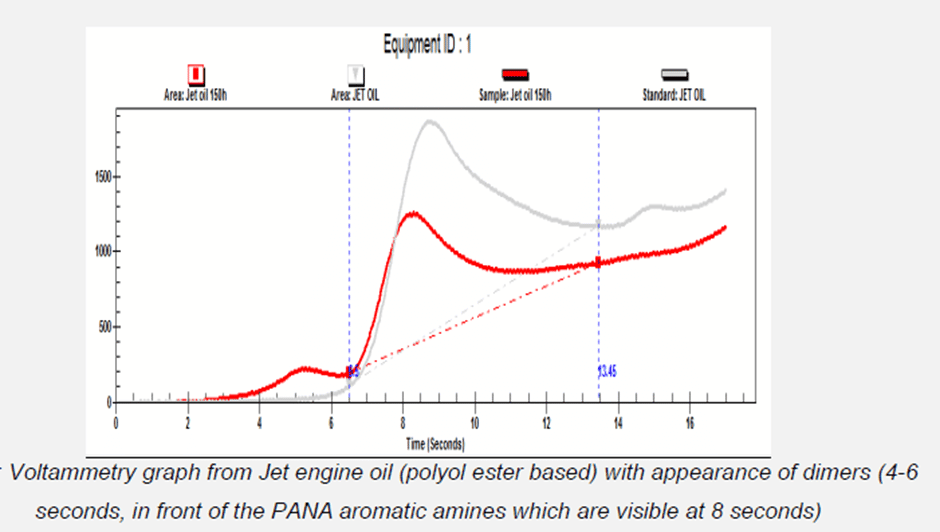
Quite contrarily, aromatic amines such as phenyl-alpha-naphtyl amine (PANA) can create dimers and trimers during oxidation. These oligomers have an electrochemical response and will appear in front of the aromatic amines as per below. Their reduced solubility will contribute to the formation of coke and other deposits hence it is important to measure these polymers.
To detect salicylates and phenates, one must first react with acid to regenerate the phenol and then analyze these similar to hindered phenols. The Blue solution was specifically developed for this and can be seen below.
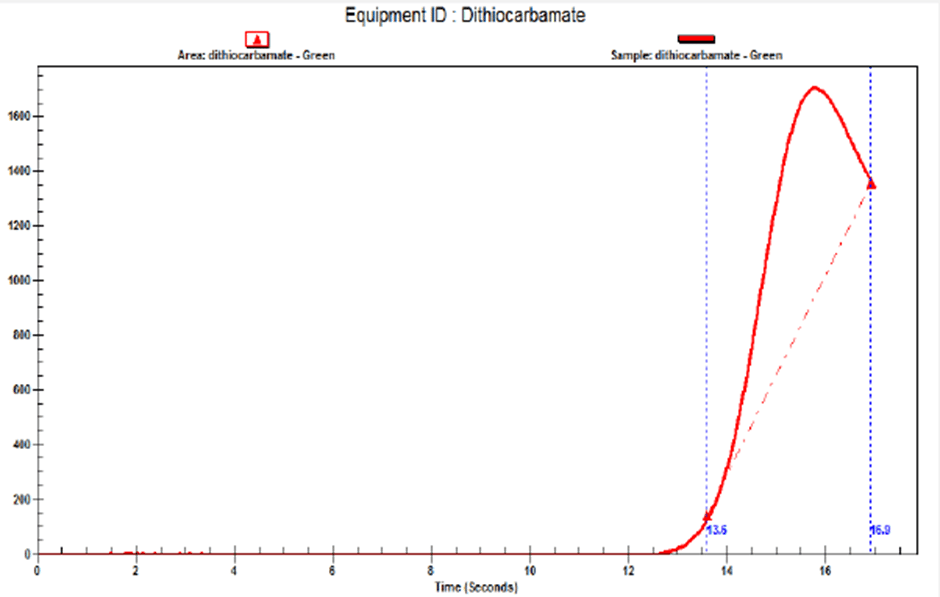
The detection of carbamates is also critical for quality control. The Green solution should be used here as carbamates will appear on the graph as shown below. The yellow solution should not be used as the carbamates do not show up.
Applications of LSV using RULER
Linear Sweep Voltammetry (LSV) is the method used in the RULER analysis and it produces quantifiable results which help us in determining the condition of the lubricant. In particular, it can be used as a guide for interpreting turbine, compressor and other R&O (Rust & Oxidation) oils as shown below:
- *Amine only formulations: the warning limit is 50% and condemning limit is 25%
- *Phenol only formulations: the warning limit is 50% and condemning limit is 25%
- *Amine – Phenol formulations: the warning limit is 50% of amines remaining and condemning limit is 25% of amines remaining
- *Amine-Phenol Depletion Ratios: during oxidation, phenols regenerate amines thereby depleting first. If the amines are depleting at the same rate or faster than phenols, then water is entering the reaction causing this unusual mode of degradation.
LSV provides the best way for remaining antioxidants to be accurately quantified through the use of the RULER equipment. As such, users can more easily predict possible degradation patterns of their lubricant and adequately plan for these instances to avoid downtime. Through the use of application specific electrolytes, the guesswork is taken out of determining the remaining useful life of the lubricant.